Your Who performed the gold foil experiment images are available in this site. Who performed the gold foil experiment are a topic that is being searched for and liked by netizens today. You can Download the Who performed the gold foil experiment files here. Download all free photos and vectors.
If you’re looking for who performed the gold foil experiment images information connected with to the who performed the gold foil experiment topic, you have come to the right site. Our site always gives you suggestions for viewing the highest quality video and picture content, please kindly surf and find more enlightening video content and graphics that match your interests.
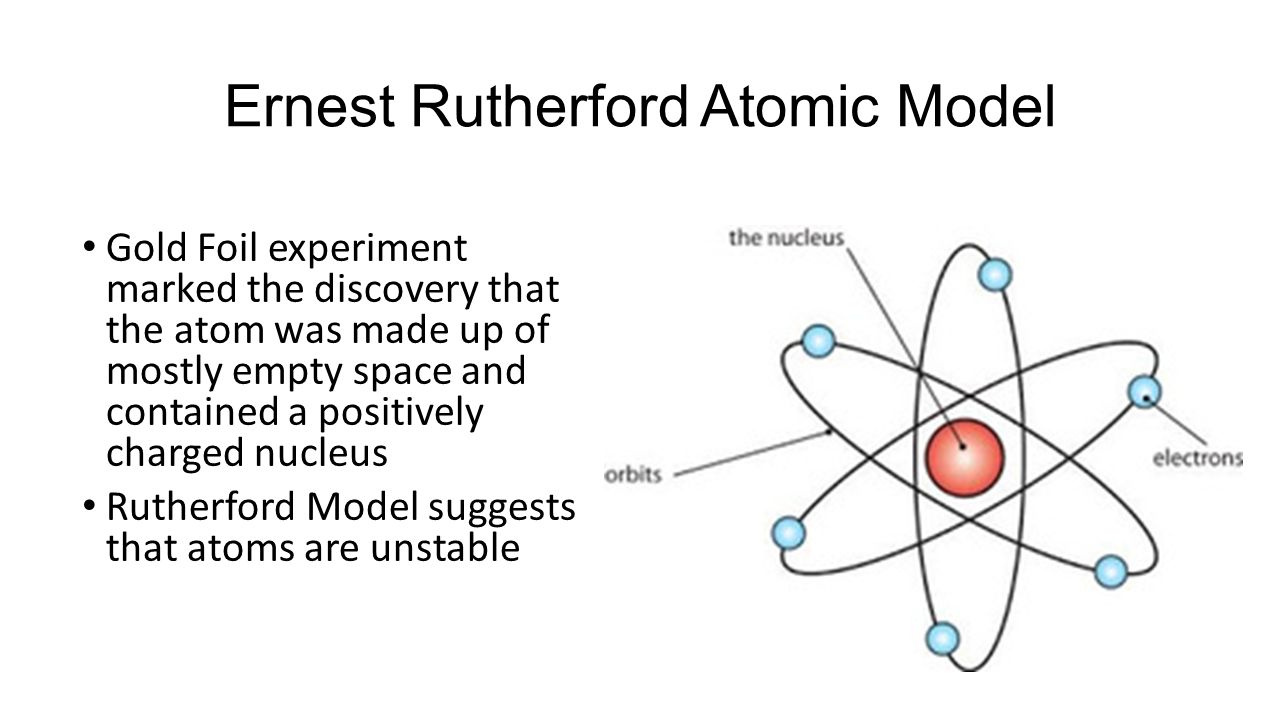
Who Performed The Gold Foil Experiment. If you were to use Mg in place of Au in the Rutherford experiment how would you predict the results from the experiment to change. Ernest Rutherford Hans Geiger and Ernest Marsden carried out their Gold Foil Experiment to observe the effect of alpha. The Rutherford gold foil experiment was performed to find out the structure of the atom. Before Rutherfords experiment the best model of the atom that was known to us was the Thomson or plum pudding model.
 Rutherford Gold Foil Experiment Google Search Ciencia From pinterest.com
Rutherford Gold Foil Experiment Google Search Ciencia From pinterest.com
The gold-foil experiment showed that the atom consists of a small massive positively charged nucleus with the negatively charged electrons being at a great distance from the centre. He realized this because most of the alpha particles passed straight through the piece of gold foil with just. Click to see full answer. Rutherfords Gold Foil Experiment Geiger-Marsden Experiment. Rutherford performed the gold foil experiment to _____. He bombarded a stream of a-particles on a gold foil a thin sheet which was 000006 cm thick in an evacuated chamber.
Before Rutherfords experiment the best model of the atom that was known to us was the Thomson or plum pudding model.
The experiment proved that the mass of an atom is concentrated in the center of the atom. Ernest Rutherford Hans Geiger and Ernest Marsden. The element magnesium has significantly fewer protons and neutrons than gold. Rutherford teamed up with his assistant Hans Geiger and Ernst Marsden who was an undergraduate student working in Rutherfords lab. An a-particle is a positively charged helium ion He. In 1911 Rutherford performed the gold foil experiment.
 Source: cz.pinterest.com
Source: cz.pinterest.com
Lesson Summary Rutherfords gold foil experiment showed that atoms are mostly empty space with the positive charge concentrated in a nucleus. Which statement regarding the gold foil experiment is false. A confirmed the plum-pudding model of the atom B led to the discovery of the atomic nucleus C was the basis for Thomsons model of the atom D utilized the deflection of beta particles by gold foil E proved the law of multiple proportions. He said about his experiment he said It was as if you fired a 15-inch shell at a. The gold foil experiment performed in Rutherfords lab _____.
 Source: in.pinterest.com
Source: in.pinterest.com
A confirmed the plum-pudding model of the atom B led to the discovery of the atomic nucleus C was the basis for Thomsons model of the atom. Rutherfords gold foil experiment. It was performed in 1909. In the experiment he emitted positively charged particles at a piece of gold foil. Rutherford teamed up with his assistant Hans Geiger and Ernst Marsden who was an undergraduate student working in Rutherfords lab.
 Source: pinterest.com
Source: pinterest.com
A It was performed by Rutherford and his research group early in the 20th century. Between 1908 and 1913 a series of experiments were performed by Hans Geiger and Ernest Marsden under the guidance of Ernest Rutherford. Rutherford in his experiment directed high energy streams of α-particles from a radioactive source at a thin sheet 100 nm thickness of gold. Bohrs model of the hydrogen atom. Rutherford teamed up with his assistant Hans Geiger and Ernst Marsden who was an undergraduate student working in Rutherfords lab.
 Source: pinterest.com
Source: pinterest.com
B Most of the alpha particles passed through the foil undeflected. The gold foil experiment consisted of a series of tests in which a positively charged helium particle was shot at a very thin layer of gold foil. Before Rutherfords experiment the best model of the atom that was known to us was the Thomson or plum pudding model. The GeigerMarsden experiments also called the Rutherford gold foil experiment were a landmark series of experiments by which scientists learned that every atom has a nucleus where all of its positive charge and most of its mass is concentrated. B Most of the alpha particles passed through the foil undeflected.
 Source: in.pinterest.com
Source: in.pinterest.com
He realized this because most of the alpha particles passed straight through the piece of gold foil with just. Rutherfords conducted an experiment by bombarding a thin sheet of gold with α-particles and then studied the trajectory of these particles after their interaction with the gold foil. Alfa particles are relatively massive particles travelling at a high speed so Rutherford thought all of the. An a-particle is a positively charged helium ion He. 35 The gold foil experiment performed in Rutherfords lab 35 A confirmed the plum-pudding model of the aton B utilized the deflection of beta particles by gold foil D led to the discovery of the atomic nucleus E proved the law of multiple proportions 36 Cathode rays are 36 A protons B X-rays C electrons D neutronsE atoms 37 All atoms of a.
 Source: pinterest.com
Source: pinterest.com
In 1911 Rutherford performed the gold foil experiment. Confirm the plum pudding model. Click to see full answer. Rutherford performed the gold foil experiment to _____. Rutherfords Gold Foil Experiment Geiger-Marsden Experiment.
 Source: in.pinterest.com
Source: in.pinterest.com
A confirmed the plum-pudding model of the atom B led to the discovery of the atomic nucleus C was the basis for Thomsons model of the atom D utilized the deflection of beta particles by gold foil E proved the law of multiple proportions. He realized this because most of the alpha particles passed straight through the piece of gold foil with just. Conducted Gold Foil Experiment also known as the Geiger-Marsden experimentHis idea was to probe the structure of Atom by firing α. Herein who performed the gold foil experiment. He unexpectedly observed a few of the particles scattered almost directly backward.
 Source: pinterest.com
Source: pinterest.com
The gold-foil experiment showed that the atom consists of a small massive positively charged nucleus with the negatively charged electrons being at a great distance from the centre. He bombarded a stream of a-particles on a gold foil a thin sheet which was 000006 cm thick in an evacuated chamber. The gold-foil experiment showed that the atom consists of a small massive positively charged nucleus with the negatively charged electrons being at a great distance from the centre. 2 Ernest Rutherford. Herein who performed the gold foil experiment.
 Source: pinterest.com
Source: pinterest.com
Rutherford teamed up with his assistant Hans Geiger and Ernst Marsden who was an undergraduate student working in Rutherfords lab. He unexpectedly observed a few of the particles scattered almost directly backward. Lesson Summary Rutherfords gold foil experiment showed that atoms are mostly empty space with the positive charge concentrated in a nucleus. In 1911 Rutherford performed the gold foil experiment. Opposite the gold foil is a screen that emits a flash of light when struck by a particle.
 Source: pinterest.com
Source: pinterest.com
Ernest Rutherford Hans Geiger and Ernest Marsden. This result was not consistent with then current models of atomic structure and led Rutherford to propose the existence of a very dense. In this model the atom was believed to consist of a positive material pudding with negative plums distributed throughout. In Bohrs model the orbits of the electrons were explained by quantum mechanics. He said about his experiment he said It was as if you fired a 15-inch shell at a.
 Source: pinterest.com
Source: pinterest.com
A confirmed the plum-pudding model of the atom B led to the discovery of the atomic nucleus C was the basis for Thomsons model of the atom D utilized the deflection of beta particles by gold foil E proved the law of multiple proportions. Also Know how does the gold foil experiment work. He bombarded a stream of a-particles on a gold foil a thin sheet which was 000006 cm thick in an evacuated chamber. Video transcript - Voiceover This is a quote by a physicist as a comment on one of his experimental results. The Rutherford gold foil experiment or alpha particles scattering experiment remains a famous experiment in the history of science.
 Source: pinterest.com
Source: pinterest.com
Conducted Gold Foil Experiment also known as the Geiger-Marsden experimentHis idea was to probe the structure of Atom by firing α. Similarly you may ask why did Rutherford perform the gold foil experiment. Also Know how does the gold foil experiment work. Ernest Rutherford Hans Geiger and Ernest Marsden. Niels Bohr built upon Rutherfords model to make his own.
 Source: pinterest.com
Source: pinterest.com
Ernest Rutherford Hans Geiger and Ernest Marsden. The GeigerMarsden experiments also called the Rutherford gold foil experiment were a landmark series of experiments by which scientists learned that every atom has a nucleus where all of its positive charge and most of its mass is concentrated. Also Know how does the gold foil experiment work. Which statement regarding the gold foil experiment is false. Niels Bohr built upon Rutherfords model to make his own.
 Source: br.pinterest.com
Source: br.pinterest.com
It also proved that an atom is mostly empty space. A It was performed by Rutherford and his research group early in the 20th century. He said about his experiment he said It was as if you fired a 15-inch shell at a. A confirmed the plum-pudding model of the atom B led to the discovery of the atomic nucleus C was the basis for Thomsons model of the atom. It also proved that an atom is mostly empty space.
 Source: pinterest.com
Source: pinterest.com
This is the currently selected item. This is the currently selected item. Rutherfords diffraction experiment tests diffraction via a thin foil made of gold metal. The experiment proved that the mass of an atom is concentrated in the center of the atom. 2 Ernest Rutherford.
 Source: pinterest.com
Source: pinterest.com
This result was not consistent with then current models of atomic structure and led Rutherford to propose the existence of a very dense. He realized this because most of the alpha particles passed straight through the piece of gold foil with just. In 1911 Rutherford performed the gold foil experiment. Before Rutherfords experiment the best model of the atom that was known to us was the Thomson or plum pudding model. He bombarded a stream of a-particles on a gold foil a thin sheet which was 000006 cm thick in an evacuated chamber.
 Source: pinterest.com
Source: pinterest.com
Conducted Gold Foil Experiment also known as the Geiger-Marsden experimentHis idea was to probe the structure of Atom by firing α. Click to see full answer. Similarly you may ask why did Rutherford perform the gold foil experiment. The gold foil experiment consisted of a series of tests in which a positively charged helium particle was shot at a very thin layer of gold foil. Rutherford teamed up with his assistant Hans Geiger and Ernst Marsden who was an undergraduate student working in Rutherfords lab.
 Source: pinterest.com
Source: pinterest.com
Rutherfords Gold Foil Experiment proved the existance of a small massive center to atoms which would later be known as the nucleus of an atom. The experiment proved that the mass of an atom is concentrated in the center of the atom. They deduced this after measuring how an alpha particle beam is scattered when it strikes a thin metal foil. The GeigerMarsden experiments also called the Rutherford gold foil experiment were a landmark series of experiments by which scientists learned that every atom has a nucleus where all of its positive charge and most of its mass is concentrated. He unexpectedly observed a few of the particles scattered almost directly backward.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title who performed the gold foil experiment by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






