Your What did rutherford expect to happen in his experiment images are available. What did rutherford expect to happen in his experiment are a topic that is being searched for and liked by netizens now. You can Download the What did rutherford expect to happen in his experiment files here. Find and Download all royalty-free vectors.
If you’re searching for what did rutherford expect to happen in his experiment pictures information connected with to the what did rutherford expect to happen in his experiment topic, you have come to the ideal blog. Our site always provides you with suggestions for refferencing the highest quality video and image content, please kindly surf and locate more enlightening video articles and images that match your interests.
What Did Rutherford Expect To Happen In His Experiment. Based on Thomsons plum pudding model Rutherford predicted that most of the α particles would pass straight through the gold foil. So think of the model as a spherical Christmas cake. In 1911 Ernest Rutherford performed an experiment to test the plum pudding model. Based on Thomsons plum pudding model Rutherford predicted that most of the α particles would pass straight through the gold foil.
 Rutherford Gold Foil Experiment Infographic Diagram Showing Deflected And Undeflected Alpha Particles When A Beam Hit Infographic Science Education Experiments From pinterest.com
Rutherford Gold Foil Experiment Infographic Diagram Showing Deflected And Undeflected Alpha Particles When A Beam Hit Infographic Science Education Experiments From pinterest.com
What did Rutherford expect to happen in his experiment. What would happen if Rutherford used negative particles. Based on Thomsons plum pudding model Rutherford predicted that most of the α particles would pass straight through the gold foil. Why did he expect this. Rutherford expected all of the particles to be deflected just a bit as they passed through the plum pudding. What did Rutherford expect to happen in his experiment quizlet.
Most alpha particles went straight through the gold foil but a few were deflected at large angles or backwards.
Electrons are many thousand times smaller than the nucleus and negatively charged. Why did he expect this. What did Ernest Rutherford expect to happen when he aimed a beam of particles at a thin gold foil the particles would deflect at sharp angles or completely back toward the emitter the particles would pass through the foil undisturbed the particles would deflect. Rutherfords experiment showed that the atom does not contain a uniform distribution of charge. Also what did Rutherford expect to happen in his experiment. What did rutherford expect would happen in his experiment.
 Source: pinterest.com
Source: pinterest.com
What did Rutherford expect to happen in his experiment. What did Rutherford expect to happen in his experiment. Based on Thomsons plum pudding model Rutherford predicted that most of the α particles would pass straight through the gold foil. He fired energetic a He 2 particles at a foil and measured the deflection of the particles as they came out the other side. Rutherford has demonstrated that the atomic nucleus is positive and separate from the electrons.
 Source: pinterest.com
Source: pinterest.com
What did Rutherford expect would happen in his experiment. What actually happened in Rutherfords experiment. This is because the positive charge in the plum pudding model was assumed to be spread out throughout the entire volume of the atom. What did Rutherford expect to happen. He noticed that a sample of radioactive material invariably took the same amount of time for half the sample to decay its half-life and created a practical application for this phenomenon using this constant rate of decay as a clock which could then be used to help determine the actual age of.
 Source: pinterest.com
Source: pinterest.com
Expected results of Rutherfords gold foil experiment. Rutherford expected all of the particles to be deflected just a bit as they passed through the plum pudding. He expected this to happen because of the Plum Pudding Model. What did Rutherford expect would happen in his experiment. What did Rutherford expect to happen in his experiment.
 Source: pinterest.com
Source: pinterest.com
What did Rutherford expect to happen when he aimed particles at the gold foil. Rutherford does not stop there he continued his researches on the properties of radioactive radiations. A plum pudding was a Christmas cake studded with raisins plums. Now imagine you have a tennis ball and you decide to throw it against that wall. Based on Thomsons plum pudding model Rutherford predicted that most of the α particles would pass straight through the gold foil.
 Source: pinterest.com
Source: pinterest.com
This is because the positive charge in the plum pudding model was assumed to be spread out throughout the entire volume of the atom. What did Rutherford expect would happen in his experiment. Alpha particles passing through the plum pudding model of the atom undisturbed. Rutherford concluded that the positive charge of the atom must be concentrated into a very small location. What 2 Things did Rutherford conclude from his experiment.
 Source: pinterest.com
Source: pinterest.com
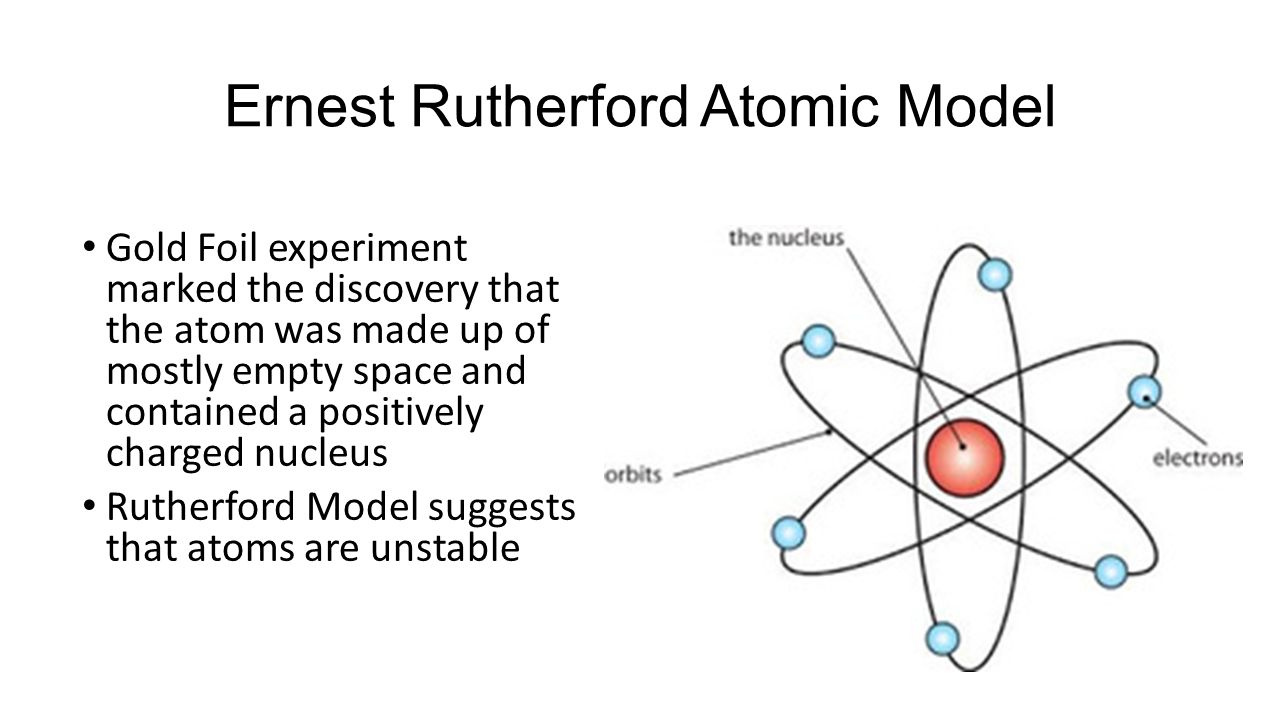
The gold-foil experiment showed that the atom consists of a small massive positively charged nucleus with the negatively charged electrons being at a great distance from the centre. What 2 Things did Rutherford conclude from his experiment. Rutherford does not stop there he continued his researches on the properties of radioactive radiations. What did Rutherford expect would happen in his experiment. Assuming a plum pudding model of the atom Rutherford predicted that the areas of positive charge in the gold atoms would deflect or bend the path of all the alpha particles as they passed through.
 Source: br.pinterest.com
Source: br.pinterest.com
Rutherfords experiment showed that the atom does not contain a uniform distribution of charge. So think of the model as a spherical Christmas cake. What did Rutherford expect to happen. Rutherford concluded that the positive charge of the atom must be concentrated into a very small location. Rutherford expected all of the particles to be deflected just a bit as they passed through the plum pudding.
 Source: pinterest.com
Source: pinterest.com
What did rutherford expect would happen in his experiment. What did Rutherford expect would happen in his experiment. Expected results of Rutherfords gold foil experiment. Assuming a plum pudding model of the atom Rutherford predicted that the areas of positive charge in the gold atoms would deflect or bend the path of all the alpha particles as they passed throughOnly a few of the alpha particles were deflected from their straight path as Rutherford had predicted. Rutherford expected all of the particles to be deflected just a bit as they passed through the plum pudding.
 Source: pinterest.com
Source: pinterest.com
A plum pudding was a Christmas cake studded with raisins plums. What 2 Things did Rutherford conclude from his experiment. Also what did Rutherford expect to happen in his experiment. What did Ernest Rutherford expect to happen when he aimed a beam of particles at a thin gold foil the particles would deflect at sharp angles or completely back toward the emitter the particles would pass through the foil undisturbed the particles would deflect. Since the previous atomic model the Thomson model argues that an atom is a sphere of positive charge with the negatively-charged electrons scattered like raisins in a pudding Rutherford and his students fully expected that an alpha particle will pass through the gold foil with just a slight deflection on the angles since the alpha particle is weakly positively.
 Source: pinterest.com
Source: pinterest.com
Thomsons plum pudding model viewed the atom as a massive blob of positive charge dotted with negative charges. He expected the alpha particles to straight through the foil because he thought the alpha particles would go straight through the foil because they are positively charged. What did Rutherford expect to happen when he aimed particles at the gold foil. Thomsons plum pudding model viewed the atom as a massive blob of positive charge dotted with negative charges. What did Ernest Rutherford expect to happen when he aimed a beam of particles at a thin gold foil the particles would deflect at sharp angles or completely back toward the emitter the particles would pass through the foil undisturbed the particles would deflect.
 Source: pinterest.com
Source: pinterest.com
Rutherford concluded that the positive charge of the atom must be concentrated into a very small location. What did Rutherford expect would happen in his experiment. Most alpha particles went straight through the gold foil but a few were deflected at large angles or backwards. Based on Thomsons plum pudding model Rutherford predicted that most of the α particles would pass straight through the gold foil. Thomsons plum pudding model viewed the atom as a massive blob of positive charge dotted with negative charges.
 Source: pinterest.com
Source: pinterest.com
Also what did Rutherford expect to happen in his experiment. Assuming a plum pudding model of the atom Rutherford predicted that the areas of positive charge in the gold atoms would deflect or bend the path of all the alpha particles as they passed throughOnly a few of the alpha particles were deflected from their straight path as Rutherford had predicted. This is because the positive charge in the plum pudding model was assumed to be spread out throughout the entire volume of the atom. What did Rutherford expect would happen in his experiment. What did rutherford expect would happen in his experiment.
 Source: pinterest.com
Source: pinterest.com
Expected results of Rutherfords gold foil experiment. What did Rutherford expect to happen when he aimed particles at the gold foil. What did Rutherfords experiment prove. He fired energetic a He 2 particles at a foil and measured the deflection of the particles as they came out the other side. Rutherford concluded that the positive charge of the atom must be concentrated into a very small location.
 Source: pinterest.com
Source: pinterest.com
Alpha particles passing through the plum pudding model of the atom undisturbed. Assuming a plum pudding model of the atom Rutherford predicted that the areas of positive charge in the gold atoms would deflect or bend the path of all the alpha particles as they passed through. Rutherford concluded that the positive charge of the atom must be concentrated into a very small location. What did Rutherford expect would happen in his experiment. Now imagine you have a tennis ball and you decide to throw it against that wall.
 Source: in.pinterest.com
Source: in.pinterest.com
Rutherford expected all of the particles to be deflected just a bit as they passed through the plum pudding. A plum pudding was a Christmas cake studded with raisins plums. Also what did Rutherford expect to happen in his experiment. What did Rutherford expect to happen in his famous experiment. Based on Thomsons plum pudding model Rutherford predicted that most of the α particles would pass straight through the gold foil.
 Source: pinterest.com
Source: pinterest.com
Rutherford does not stop there he continued his researches on the properties of radioactive radiations. What did Rutherford expect to happen in his experiment quizlet. Also what did Rutherford expect to happen in his experiment. Now imagine you have a tennis ball and you decide to throw it against that wall. Rutherford concluded that the positive charge of the atom must be concentrated into a very small location.
 Source: pinterest.com
Source: pinterest.com
What did Rutherford expect to happen in his experiment. What would happen if Rutherford used negative particles. This is because the positive charge in the plum pudding model was assumed to be spread out throughout the entire volume of the atom. Expected results of Rutherfords gold foil experiment. Assuming a plum pudding model of the atom Rutherford predicted that the areas of positive charge in the gold atoms would deflect or bend the path of all the alpha particles as they passed through.
 Source: pinterest.com
Source: pinterest.com
What did Rutherford expect would happen in his experiment. Rutherford has demonstrated that the atomic nucleus is positive and separate from the electrons. Thomsons plum pudding model viewed the atom as a massive blob of positive charge dotted with negative charges. What did Rutherford expect would happen in his experiment. Expected results of Rutherfords gold foil experiment.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title what did rutherford expect to happen in his experiment by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.