Your Describe rutherfords gold foil experiment images are available in this site. Describe rutherfords gold foil experiment are a topic that is being searched for and liked by netizens now. You can Download the Describe rutherfords gold foil experiment files here. Get all royalty-free photos and vectors.
If you’re looking for describe rutherfords gold foil experiment images information linked to the describe rutherfords gold foil experiment interest, you have visit the ideal site. Our site always gives you hints for downloading the highest quality video and image content, please kindly hunt and locate more informative video content and graphics that match your interests.
Describe Rutherfords Gold Foil Experiment. Khan Academy is a 501c3 nonprofit organization. The GeigerMarsden experiments also called the Rutherford gold foil experiment were a landmark series of experiments by which scientists learned that every atom has a nucleus where all of its positive charge and most of its mass is concentrated. He realized this because most of the alpha particles passed straight. How did these results refute the plum pudding model of the atom.
 3 4 Rutherford S Experiment The Nuclear Model Of The Atom Chemistry Libretexts From chem.libretexts.org
3 4 Rutherford S Experiment The Nuclear Model Of The Atom Chemistry Libretexts From chem.libretexts.org
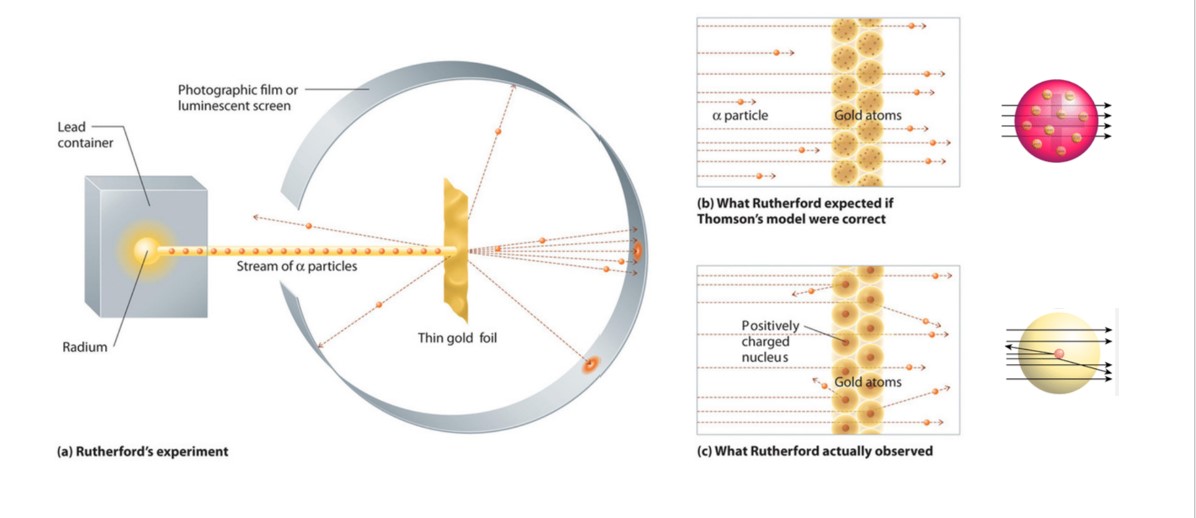
They deduced this after measuring how an alpha particle beam is scattered when it strikes a thin metal foil. The GeigerMarsden experiments also called the Rutherford gold foil experiment were a landmark series of experiments by which scientists learned that every atom has a nucleus where all of its positive charge and most of its mass is concentrated. Prior to the groundbreaking gold foil experiment Rutherford was granted the Nobel Prize for other key contributions in. They bombarded fragile sheets of gold foil with fast-moving alpha particles. The Rutherford Gold Foil Experiment offered the first experimental evidence that led to the discovery of the nucleus of the atom as a small dense and positively charged atomic core. He also proposed the position and behaviour of electrons.
The essential idea of Rutherfords theory is to consider the -particle as a charged mass traveling.
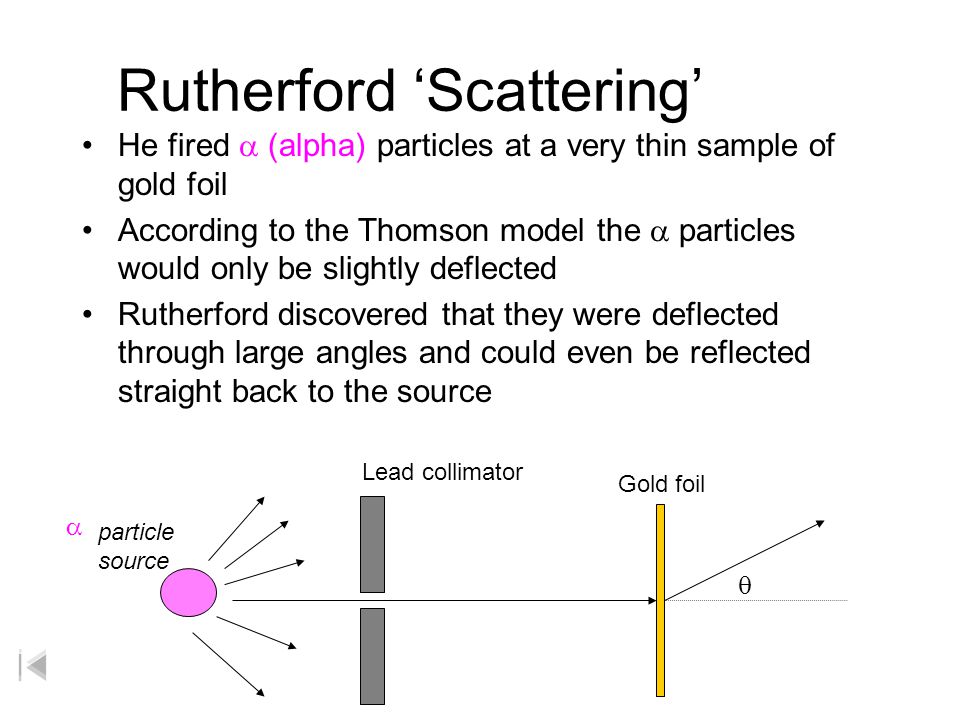
Rutherfords gold foil experiment involved sending positively charged alpha particles through a thin sheet of gold foil and defecting if there was any deflection of the particles. In 1905 Ernest Rutherford did an experiment to test the plum pudding model. The evidence for the existence of the nucleus has been provided by Rutherfords Experiment which he carried out in 1911. In the experiment Rutherford and his two students studied how alpha particles fired at a thin piece of gold foil were deflected. The GeigerMarsden experiments also called the Rutherford gold foil experiment were a landmark series of experiments by which scientists learned that every atom has a nucleus where all of its positive charge and most of its mass is concentrated. Alpha beam and this can be covered with gold foil to fully simulate the energy loss in the gold foil scattering experiment.
 Source: quora.com
Source: quora.com
He conduct an experiment by bombarding alpha particles into a thin sheet of gold and then notices their interaction with the gold foil and trajectory or path followed by these particles. The Rutherford gold foil experiment or alpha particles scattering experiment remains a famous experiment in the history of science. He bombarded the gold foil with alpha rays. Describe Rutherfords gold foil experiment and how the experimental results led to specific changes in the accepted model of the atom. The Rutherford model is a model of the atom devised by Ernest RutherfordRutherford directed the famous GeigerMarsden experiment in 1909 which suggested upon Rutherfords 1911 analysis that J.

Niels Bohr built upon Rutherfords model to make his own. The gold-foil experiment showed that the atom consists of a small massive positively charged nucleus with the negatively charged electrons being at a great distance from the centre. Thomsons plum pudding model of the atom was incorrect. Rutherfords gold foil experiment Rutherfords alpha particle scattering experiment refers to an experiment carried out by Ernest Rutherford Hans Geiger and Ernest Marsden at the University of Manchester in the early 1900s. This region would be known as the nucleus of the atom.
 Source: large.stanford.edu
Source: large.stanford.edu
The GeigerMarsden experiments also called the Rutherford gold foil experiment were a landmark series of experiments by which scientists learned that every atom has a nucleus where all of its positive charge and most of its mass is concentrated. The experiment began in 1908. This region would be known as the nucleus of the atom. 2 Very few particles deflected from their path this shows that positive charge occupies less space. The Rutherford Gold Foil Experiment offered the first experimental evidence that led to the discovery of the nucleus of the atom as a small dense and positively charged atomic core.
 Source: slideplayer.com
Source: slideplayer.com
1 Most of the space inside the atom is empty as a-particles passed through the foil. Most of the time the alpha particles would pass through the foil without any change in their trajectories which is what was expected if JJ. The gold foil experiment results in the Rutherford model where the atom is composed of a positively charged nucleus surrounded by negatively charged electrons. Alpha beam and this can be covered with gold foil to fully simulate the energy loss in the gold foil scattering experiment. How did these results refute the plum pudding model of the atom.
 Source: zapscience.com
Source: zapscience.com
He bombarded the gold foil with alpha rays. The third was Hans Geiger. 2 Very few particles deflected from their path this shows that positive charge occupies less space. Rutherfords gold foil experiment led to the discovery that most of an atoms mass is located in a dense region now called the nucleus. They deduced this after measuring how an alpha particle beam is scattered when it strikes a thin metal foil.
 Source: flexbooks.ck12.org
Source: flexbooks.ck12.org
They deduced this after measuring how an alpha particle beam is scattered when it strikes a thin metal foil. How did these results refute the plum pudding model of the atom. Rutherfords Alpha Scattering Experiment. They bombarded fragile sheets of gold foil with fast-moving alpha particles. Observations of Rutherfords alpha ray scattering experiment.
 Source: studylib.net
Source: studylib.net
Some of the α-particles were deflected by the foil by some angles. He beamed a ray of alpha particles onto a gold foil and. 1 Most of the space inside the atom is empty as a-particles passed through the foil. He realized this because most of the alpha particles passed straight. He conduct an experiment by bombarding alpha particles into a thin sheet of gold and then notices their interaction with the gold foil and trajectory or path followed by these particles.
 Source: expii.com
Source: expii.com
Rutherford in 1911 carried out an experiment called Gold foil experiment and could conclude the nature of an atom and the position of the protons present in the atom decisively. Ernest Rutherford Hans Geiger and Ernest Marsden. Rutherford passed beams of alpha particles through a thin gold foil and noted how the alpha particles scattered from the foil. He bombarded the gold foil with alpha rays. He realized this because most of the alpha particles passed straight.
 Source: chem.libretexts.org
Source: chem.libretexts.org
Most of the time the alpha particles would pass through the foil without any change in their trajectories which is what was expected if JJ. Describe Rutherfords gold foil experiment and how the experimental results led to specific changes in the accepted model of the atom. Most of the α-particles passed straight through the gold foil without any deviation. In Bohrs model the orbits of the electrons were explained by quantum mechanics. The gold-foil experiment showed that the atom consists of a small massive positively charged nucleus with the negatively charged electrons being at a great distance from the centre.

The alpha particles that were fired at the gold foil were positively charged. Niels Bohr built upon Rutherfords model to make his own. This chemistry video tutorial provides a basic introduction into Rutherfords Gold Foil Experiment. He also proposed the position and behaviour of electrons. Ernest Rutherford Hans Geiger and Ernest Marsden.
 Source: chemistrygod.com
Source: chemistrygod.com
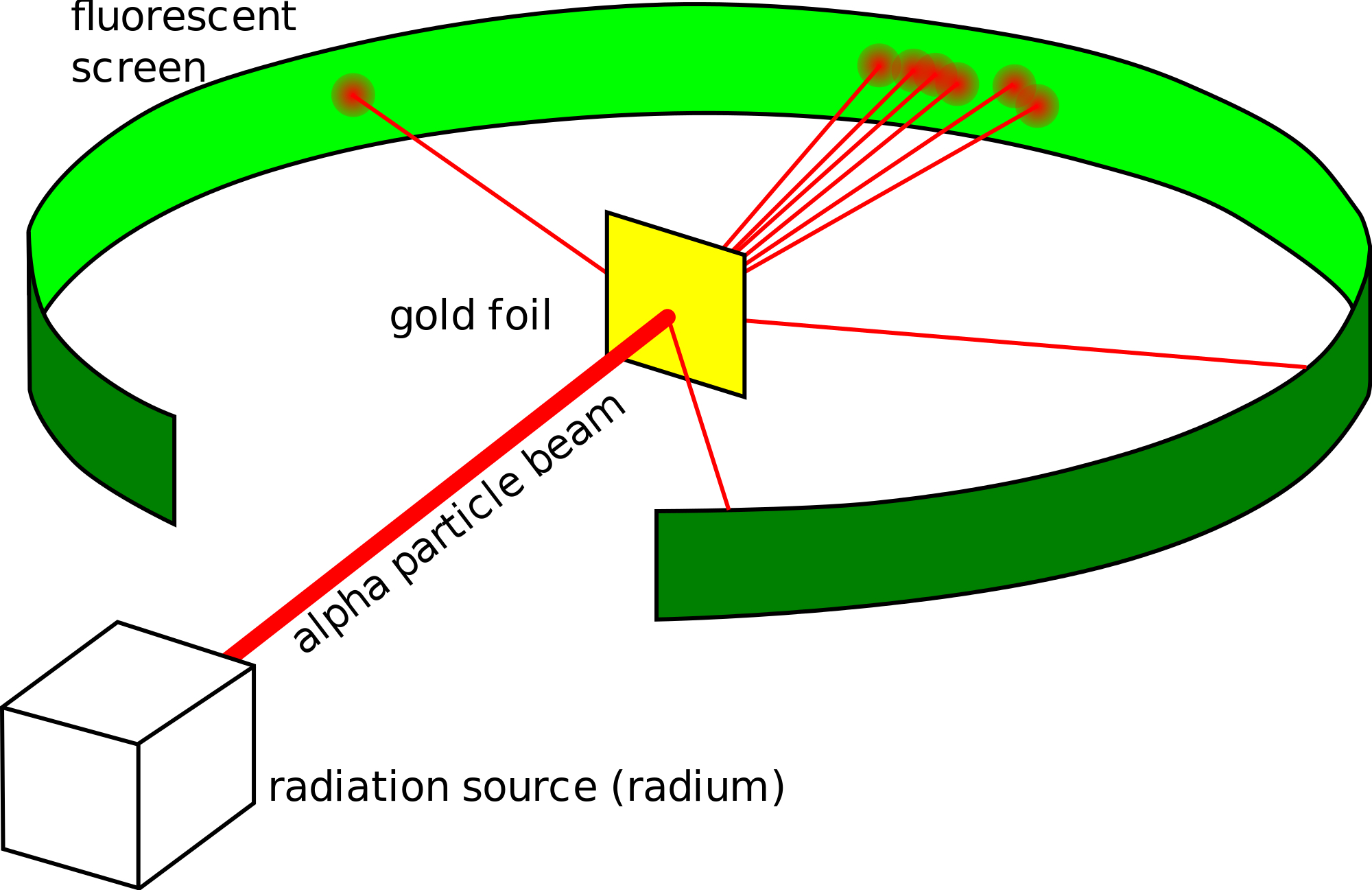
This image shows the cage with alpha source and gold scattering foil prepared for a. The experiment began in 1908. Based on the above activity and similar reasoning Rutherford concluded the a-particle scattering experiment as. He conduct an experiment by bombarding alpha particles into a thin sheet of gold and then notices their interaction with the gold foil and trajectory or path followed by these particles. Rutherfords gold foil experiment showed that atoms are mostly empty space with the positive charge concentrated in a nucleus.
 Source: sites.google.com
Source: sites.google.com
There were three people involved in the Gold Foil Experiment including Ernest Marsden and Ernest Rutherford. Rutherfords gold foil experiment showed that atoms are mostly empty space with the positive charge concentrated in a nucleus. In Bohrs model the orbits of the electrons were explained by quantum mechanics. Rutherfords gold foil experiment involved sending positively charged alpha particles through a thin sheet of gold foil and defecting if there was any deflection of the particles. Rutherfords Gold Foil Experiment.
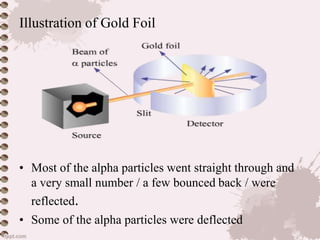 Source: slideshare.net
Source: slideshare.net
Niels Bohr built upon Rutherfords model to make his own. The Rutherford model is a model of the atom devised by Ernest RutherfordRutherford directed the famous GeigerMarsden experiment in 1909 which suggested upon Rutherfords 1911 analysis that J. Observations of Rutherfords alpha ray scattering experiment. Rutherford passed beams of alpha particles through a thin gold foil and noted how the alpha particles scattered from the foil. The essential idea of Rutherfords theory is to consider the -particle as a charged mass traveling.
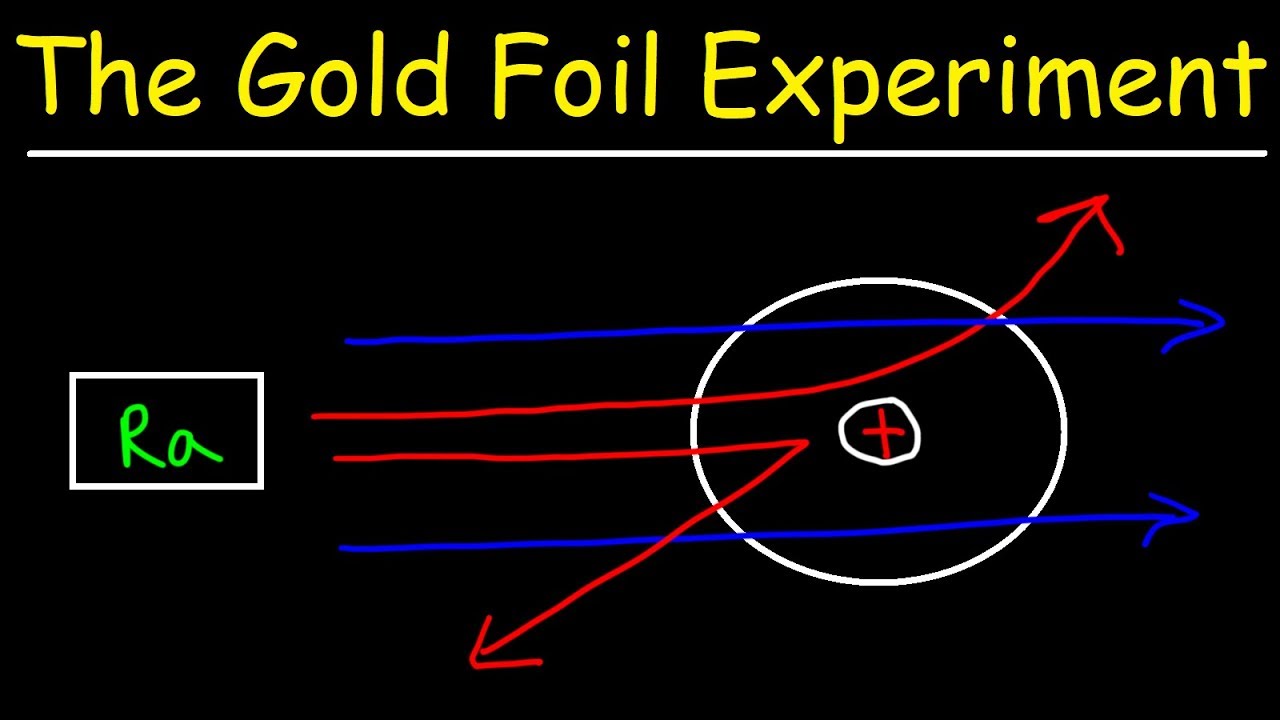 Source: youtube.com
Source: youtube.com
Observations of Rutherfords alpha ray scattering experiment. Khan Academy is a 501c3 nonprofit organization. He also proposed the position and behaviour of electrons. The essential idea of Rutherfords theory is to consider the -particle as a charged mass traveling. Be sure to include a description of the atomic model that was accepted by the scientific community before this experiment as well as the atomic model that was accepted after this experiment.
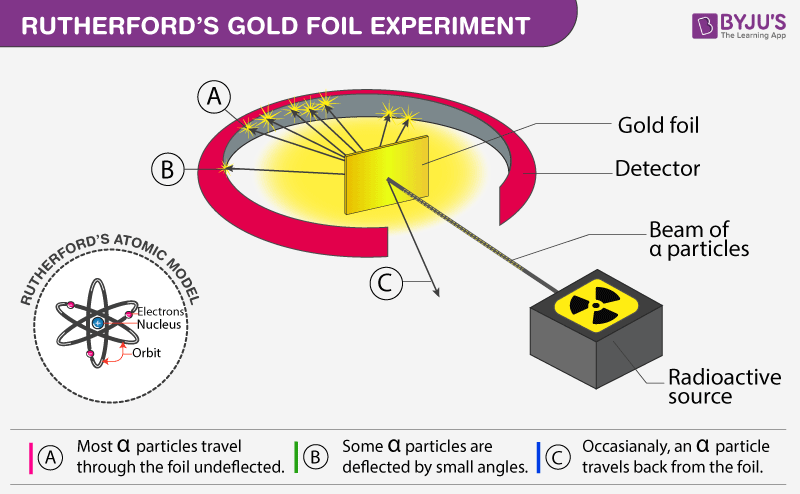 Source: byjus.com
Source: byjus.com
Observations of Rutherfords alpha ray scattering experiment. The experiment began in 1908. The Rutherford gold foil experiment or alpha particles scattering experiment remains a famous experiment in the history of science. Ernest Rutherford Hans Geiger and Ernest Marsden. The gold-foil experiment showed that the atom consists of a small massive positively charged nucleus with the negatively charged electrons being at a great distance from the centre.
 Source: study.com
Source: study.com
They deduced this after measuring how an alpha particle beam is scattered when it strikes a thin metal foil. He also proposed the position and behaviour of electrons. Rutherfords gold foil experiments and other metal foil experiments involved firing positively charged alpha particles at a piece of goldmetal foil. Rutherford passed beams of alpha particles through a thin gold foil and noted how the alpha particles scattered from the foil. He beamed a ray of alpha particles onto a gold foil and.
 Source: pinterest.com
Source: pinterest.com
The GeigerMarsden experiments also called the Rutherford gold foil experiment were a landmark series of experiments by which scientists learned that every atom has a nucleus where all of its positive charge and most of its mass is concentrated. Rutherford took a very thin gold foil with 100 nm thickness. This image shows the cage with alpha source and gold scattering foil prepared for a. In the experiment Rutherford and his two students studied how alpha particles fired at a thin piece of gold foil were deflected. Thomsons plum pudding model of the atom was incorrect.
 Source: electricalfundablog.com
Source: electricalfundablog.com
How did these results refute the plum pudding model of the atom. Thomsons plum pudding model of the atom was incorrect. Rutherfords Alpha Scattering Experiment. 2 Very few particles deflected from their path this shows that positive charge occupies less space. The Rutherford Gold Foil Experiment offered the first experimental evidence that led to the discovery of the nucleus of the atom as a small dense and positively charged atomic core.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title describe rutherfords gold foil experiment by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.